|
Ambidentate ligands
are distinguished by using the {!I}
annotation format.
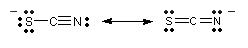
For example, the thiocyanate
(rhodanide) anion has the resonance forms
|
|
|
|
which can be encoded as ligands by indicating the donating
atom respectively:
|
|
[S-]{!I}C#N
|
S=C=[N-]{!I}
|
|
linkage via sulfur atom
|
linkage via nitrogen atom
|
|
The left notation encodes a thiocyanate ligand that interacts,
for example with a metal atom M, via the S atom, whereas
the right notation indicates interaction via the N atom.
The complex compound
K2Hg(SCN)4
is known to have the Hg-S bonding mode. This complex salt is
encoded in CurlySMILES as
|
|
[K+].[K+].[Hg+2]{+Lc=[S-]{!I}C#N{4}}
|
|
K2Hg(SCN)4
, bonding mode: M-S-C≡N
|
|
|
The complex compound
K2Co(SCN)4
is known to have the Co-N bonding mode. This complex salt is
encoded in CurlySMILES as
|
|
[K+].[K+].[Co+2]{+Lc=[S-]C#N{!I}{4}}
|
|
K2Co(SCN)4
, bonding mode: M-N=C=S
|
|
_
__
__
 __
__
__
Share on Tumblr
___
|
 __
__